Fly_dragonfly/iStock by way of Getty Photographs
I knew Zevra Therapeutics (NASDAQ:ZVRA) when it was KemPharm, a business stage developer of prodrugs. Zevra, alternatively, develops therapies for uncommon illnesses. In Could final 12 months, Zevra, then KemPharm, acquired arimoclomol, an orally-delivered, first-in-class molecule focusing on Niemann-Choose sort C illness (“NPC”), and morphed into a uncommon illness participant. Arimoclomol is a late stage product with a accomplished section 3 and the corporate is about to submit an NDA.
The pipeline appears like this:
Zevra pipeline (Zevra web site)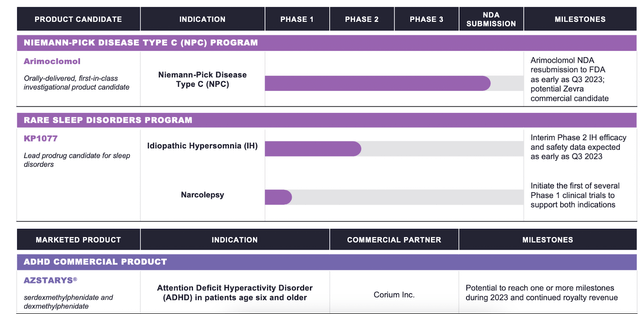
Arimoclomol will resubmit an NDA to the FDA “as early as in Q3, 2023,” says the corporate. There are 1800 US sufferers for NPC, and no authorised therapies. The asset has orphan drug designation, quick observe designation, and uncommon pediatric illness designation for NPC by the European Medicines Company (EMA) and FDA as a breakthrough remedy in NPC. Word that the mum or dad firm withdrew a Advertising Authorization Software from the EMA final 12 months, citing the “EMA’s considerations and their estimate that these couldn’t be addressed within the time out there.”
Arimoclomol was developed greater than 20 years in the past by Hungarian scientists and was thought to have therapeutic results on quite a lot of illnesses, together with ALS. In 2021, it failed an ALS section 3 trial, failing on all endpoints, though remaining secure and well-tolerated. In 2021, Orphazyme, the earlier proprietor of arimoclomol, acquired a CRL for the drug for an NDA for NPC. The CRL requested for extra knowledge:
The FDA issued the CRL based mostly on needing further qualitative and quantitative proof to additional substantiate the validity and interpretation of the 5-domain NPC Medical Severity Scale (NPCCSS) and, specifically, the swallow area. Additional, the FDA famous within the CRL that further knowledge are wanted to bolster confirmatory proof past the one section 2/3 scientific trial to assist the benefit-risk evaluation of the NDA.
A Kind A gathering held later that 12 months resulted within the following take-aways:
The FDA advisable that the Firm submit further knowledge, info, and analyses to deal with sure subjects within the CRL and interact in additional interactions with the FDA to determine a pathway to resubmission.
The FDA concurred with the Firm’s proposal to take away the cognition area from the NPCCSS endpoint, with the consequence that the first endpoint is permitted to be recalculated utilizing the 4-domain NPCCSS, topic to the submission of further requested info which the Firm intends to supply. To bolster the confirmatory proof already submitted, the FDA affirmed that it might require further in vivo or pharmacodynamic (PD)/pharmacokinetic (PK) knowledge; the Firm is contemplating the optimum path ahead to deal with the FDA’s requests.
After shopping for the asset, Zevra continued with an open label 4 12 months extension research, and just lately produced some interim evaluation in two posters on the World Symposium. I couldn’t find the precise abstracts, however the firm mentioned that knowledge confirmed that arimoclomol might presumably cut back the long run development of NPC. The corporate plans to make use of this knowledge as a part of the NDA resubmission. Curiously, this knowledge used a 5-domain NPCCSS, which, as I simply famous, has been discarded for a 4-domain NPCCSS scale.
In 2021, the corporate revealed section 2/3 security and efficacy knowledge from a trial in NPC. This was the premise of the next NDA. The trial’s knowledge was as follows:
The first endpoint was change in 5-domain NPC Medical Severity Scale (NPCCSS) rating from baseline to 12 months. Fifty sufferers enrolled; 42 accomplished. At month 12, the imply development from baseline within the 5-domain NPCCSS was 0.76 with arimoclomol vs 2.15 with placebo. A statistically important therapy distinction in favour of arimoclomol of -1.40 (95% confidence interval: -2.76, -0.03; P = .046) was noticed, akin to a 65% discount in annual illness development. Within the prespecified subgroup of sufferers receiving miglustat as routine care, arimoclomol resulted in stabilisation of illness severity over 12 months with a therapy distinction of -2.06 in favour of arimoclomol (P = .006). Hostile occasions occurred in 30/34 sufferers (88.2%) receiving arimoclomol and 12/16 (75.0%) receiving placebo. Fewer sufferers had severe antagonistic occasions with arimoclomol (5/34, 14.7%) vs placebo (5/16, 31.3%). Remedy-related severe antagonistic occasions (n = 2) included urticaria and angioedema. Arimoclomol offered a major and clinically significant therapy impact in NPC and was effectively tolerated.
The corporate’s second asset, a legacy prodrug, is KP1077, focusing on uncommon sleep indications. It has an ongoing section 2 trial in idiopathic hypersomnia, which is able to topline in 2023. A second program is narcolepsy, the place an IND has been “opened.” The corporate says {that a} constructive section 2 IH trial could lead on to a section 3 narcolepsy trial, leaping a number of steps.
Their third product is AZSTARYS®, authorised in March 2021 for ADHD in sufferers 6 years or older. The product is licensed to Corium.
Financials
ZRVA has a market cap of $186mn and a money reserve of $95mn. Analysis and growth (R&D) bills have been $8.8 million for Q1 2023, whereas basic and administrative (G&A) bills have been $6.8 million. At that price, they’ve money for some 4-5 extra quarters.
As with such outdated molecules, I at all times search for patent phrases. Right here, the info offered by the corporate is obscure. It doesn’t say what kinds of patents and by which jurisdictions have 2029 expiry:
We have now moreover acquired technique of use and technique of therapy patents, and have filed associated patent purposes, associated to the arimoclomol households (pursuant to the latest acquisition of Orphazyme) in varied jurisdictions, together with the US, European international locations, Israel, Japan, South Korea, Canada, China, Brazil, Russia and Turkey, with anticipated patent expiration date of 2029, excluding any potential patent time period changes or extensions. We anticipate submitting further patent purposes associated to the arimoclomol households.
I’m guessing US patent safety shouldn’t be for lengthy.
Dangers
I’m averse to firms with such outdated merchandise that they’re making an attempt to get to the market after a number of failures. An inertia units in, and these merchandise solely assist administration earn salaries for some extra time. This will not at all times be true, however I imagine that for these firms, there is a heavy burden of proof.
Money place and presumably patent runway are additionally insufficient.
Bottomline
I see nothing right here. There could also be some upside if Zevra submits an NDA, however I are inclined to keep away from these kinds of firms.








