Picture credit: Aer Therapeutics
Aer Therapeutics, a biopharmaceutical spin-out firm and a collaboration between College School Dublin (UCD) and the College of California, San Francisco (UCSF), introduced on Friday that it has secured $36M (roughly €32.7M) in a Sequence A spherical of funding.
The capital was acquired from a syndicate of premier life science business buyers, together with Canaan, OrbiMed, and Hatteras Enterprise Companions.
Fund utilisation
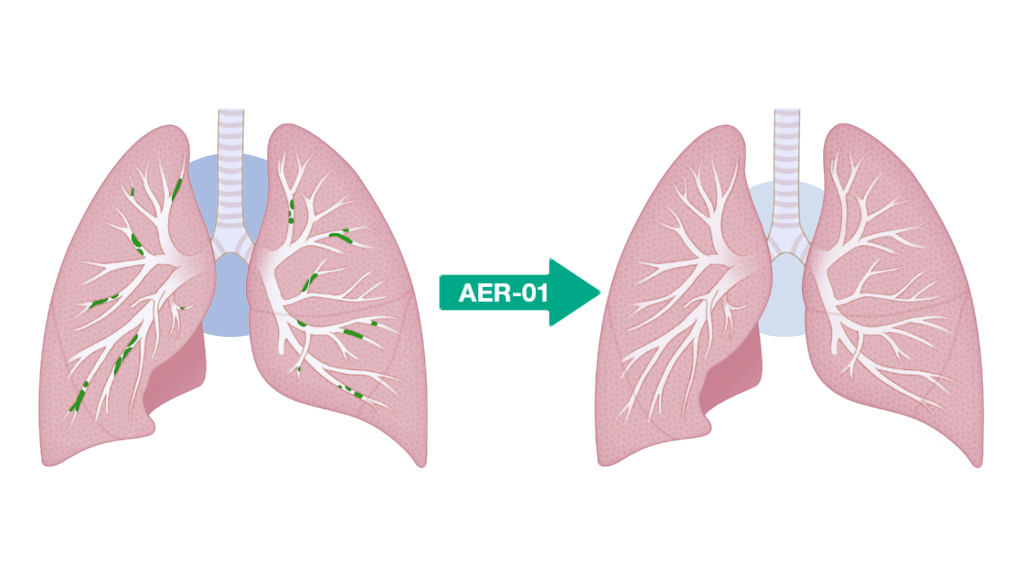
Aer Therapeutics says it is going to use the proceeds to additional develop AER-01, the corporate’s inhaled small molecule mucolytic drug designed to liquefy mucus plugs in sufferers with power obstructive pulmonary illness (COPD).
The corporate plans to conduct a first-in-human Section 1 medical trial of AER-01 in mid-2023.
Jim Shaffer, President and CEO of Aer Therapeutics, says, “We’re excited to introduce Aer Therapeutics as an organization devoted to delivering a therapeutic resolution to sufferers with COPD who’ve extreme airway obstruction brought on by mucus plugs.”
![]()
“Aer will proceed to leverage this experience within the improvement of AER-01 and different therapeutic candidates for the remedy of muco-obstructive lung illnesses,” provides Shaffer.
As a part of the Sequence A financing, the corporate has expanded its board of administrators to incorporate the next new appointees:
Tim Shannon, M.D., normal accomplice at Canaan Rishi Gupta, J.D., accomplice at OrbiMed Christy Shaffer, Ph.D., normal accomplice at Hatteras Enterprise Companions Thomas Mathers, CEO at Allievex
Therapy for mucus-associated lung illnesses
Aer Therapeutics was co-founded by Professor John Fahy, UCSF, initially from Dublin, and Professor Stefan Oscarson, UCD College of Chemistry.
Aer Therapeutics is a medical stage biopharmaceutical firm that goals to develop novel therapies for mucus-associated lung illnesses.
Professor Fahy’s laboratory at UCSF developed AER-01 with Professor Oscarson’s glycochemistry laboratory at UCD in collaboration with Professor Anne Marie Healy’s pharmaceutical know-how laboratory at Trinity School Dublin.
The mixed experience of those laboratories in mucus biology, glycochemistry, inhaled drug formulation, drug supply, and lung imaging, supported by a improvement grant from the Nationwide Institutes of Well being, underlies the novel AER-01 know-how.
Section 1 medical trials of its lead drug candidate AER-01 will begin in 2023 with the potential to broaden improvement into a number of different indications equivalent to bronchial asthma, bronchiectasis, and cystic fibrosis.
What’s AER-01?
AER-01 is a thiol-modified carbohydrate (“thiol-saccharide”) that cleaves mucin disulfide bridges to liquefy (“lyse”) mucus plugs.
Carbohydrate scaffolds are pure and non-toxic – their polar nature and excessive aqueous solubility permit them to simply penetrate mucus plugs.
AER-01 is a potent and fast-acting mucolytic well-suited for each nebulizer and dry powder supply.
John Fahy, M.D., M.Sc., Professor of Medication at UCSF and founding father of Aer Therapeutics, says, “Research utilizing CT lung scans verify that mucus plugs are extremely prevalent in COPD sufferers, and people with a excessive mucus plug burden have decrease lung perform, elevated frequency of exacerbations, diminished high quality of life, and elevated threat of all-cause mortality.”
“These findings present a foundation to particularly deal with and take away mucus plugs as a technique to enhance lung well being for COPD sufferers,” provides Fahy.
The compound is supported by a broad preclinical information bundle generated with the help of a five-year translational programme mission grant (tPPG) to Drs. Fahy, Oscarson, and Healy from the Nationwide Institutes of Well being (Nationwide Coronary heart Lung and Blood Institute).
Professor Stefan Oscarson, UCD College of Chemistry and co-founder of Aer Therapeutics, says, “It’s thrilling instances for me when after many years of educational analysis involving drug and vaccine improvement with colleagues at UCD, UCSF, and TCD, to see a lead drug candidate transferring into human medical trials. The AER-01 mucolytic drug has the potential to fulfill a variety of medical wants and to make a distinction to many lives.”








